Multiple Choice
A 25.0 mL sample of 0.150 M acetic acid is titrated with a 0.150 M NaOH solution.What is the pH at the equivalence point? The 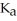 of acetic acid is 4.50 × 10-4.
of acetic acid is 4.50 × 10-4.
A) 11.74
B) 9.26
C) 4.74
D) 7.00
E) 8.81
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q84: The solubility of lead (II)chloride (PbCl<sub>2</sub>)is <img
Q85: Calculate the pH of a solution prepared
Q86: The K<sub>a</sub> of benzoic acid is 6.30
Q87: Determine the K<sub>sp</sub> for magnesium hydroxide (Mg(OH)<sub>2</sub>)where
Q88: A buffer solution with a pH of
Q90: In which of the following aqueous solutions
Q91: Which solution has the greatest buffering capacity?<br>A)0.335
Q92: What change will be caused by addition
Q93: The addition of HF and _ to
Q94: Calculate the pH of a solution that