Multiple Choice
Calculate the pH of a solution prepared by dissolving 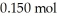 of acetic acid and
of acetic acid and 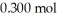 of sodium acetate in water sufficient to yield
of sodium acetate in water sufficient to yield 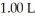 of solution.The Ka of acetic acid is
of solution.The Ka of acetic acid is 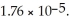
A) 2.516
B) 3.892
C) 4.502
D) 10.158
E) 5.056
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q28: The K<sub>sp</sub> for Cu(OH)<sub>2</sub> is 4.8 ×
Q29: AgBr would have the lowest solubility in
Q30: Calculate the pH of a buffer solution
Q31: A solution is prepared by dissolving 0.23
Q32: How many milliliters of 0.120 M NaOH
Q34: A 25.0 mL sample of an HCl
Q35: Which solution would have the greatest buffering
Q36: The pH of a solution prepared by
Q37: In which one of the following solutions
Q38: The pH of a solution prepared by