Multiple Choice
A solution is prepared by dissolving 0.23 mol of hypochlorous acid and 0.27 mol of sodium hypochlorite in water sufficient to yield 1.00 L of solution.The addition of 0.05 mol of HCl to this buffer solution causes the pH to drop slightly.The pH does not decrease drastically because the HCl reacts with the ________ present in the buffer solution.The 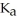 of hypochlorous acid is 1.36 × 10-3.
of hypochlorous acid is 1.36 × 10-3.
A) 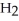 O
O
B) 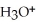
C) hypochlorite ion
D) hypochlorous acid
E) This is a buffer solution: the pH does not change upon addition of acid or base.
Correct Answer:

Verified
Correct Answer:
Verified
Q26: In a solution,when the concentrations of a
Q27: Decreasing the pH of blood will cause
Q28: The K<sub>sp</sub> for Cu(OH)<sub>2</sub> is 4.8 ×
Q29: AgBr would have the lowest solubility in
Q30: Calculate the pH of a buffer solution
Q32: How many milliliters of 0.120 M NaOH
Q33: Calculate the pH of a solution prepared
Q34: A 25.0 mL sample of an HCl
Q35: Which solution would have the greatest buffering
Q36: The pH of a solution prepared by