Multiple Choice
A 50.0 mL sample of an aqueous H2SO4 solution is titrated with a 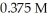 NaOH solution.The equivalence point is reached with
NaOH solution.The equivalence point is reached with 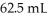 of the base.The concentration of H2SO4 is ________ M.
of the base.The concentration of H2SO4 is ________ M.
A) 0.234
B) 0.469
C) 0.150
D) 0.300
E) 0.938
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: Which compound listed below has the greatest
Q13: A 25.0 mL sample of 0.723 M
Q14: A solution containing which one of the
Q15: What is the molar solubility of silver
Q16: CaCO<sub>3</sub> is very soluble in the presence
Q18: The Henderson-Hasselbalch equation is _.<br>A)[H<sup>+</sup>] = K<sub>a</sub>
Q19: Consider a solution containing 0.100 M fluoride
Q20: A 25.0 mL sample of a solution
Q21: A 25.0-mL sample of 0.150 M hydrocyanic
Q22: For which salt should the aqueous solubility