Multiple Choice
A 25.0 mL sample of 0.723 M HCl 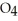 is titrated with a
is titrated with a 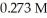 KOH solution.The
KOH solution.The 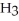
 concentration after the addition of
concentration after the addition of 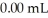 of KOH is ________ M.
of KOH is ________ M.
A) 0.0181
B) 0.430
C) 0.723
D) 0.273
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: Calculate the maximum concentration (in M)of calcium
Q9: The _ and _ are the principal
Q10: A buffer solution with a pH of
Q11: A 25.0 mL sample of a solution
Q12: Which compound listed below has the greatest
Q14: A solution containing which one of the
Q15: What is the molar solubility of silver
Q16: CaCO<sub>3</sub> is very soluble in the presence
Q17: A 50.0 mL sample of an aqueous
Q18: The Henderson-Hasselbalch equation is _.<br>A)[H<sup>+</sup>] = K<sub>a</sub>