Multiple Choice
The concentration of iodide ions in a saturated solution of lead (II) iodide is ________ M.The solubility product constant of PbI2 is 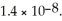
A) 3.8 × 10-4
B) 3.0 × 10-3
C) 1.5 × 10-3
D) 3.5 × 10-9
E) 1.4 × 10-8
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q106: What is the pH of a buffer
Q107: Which of the following could be added
Q108: In general,the solubility of a slightly soluble
Q109: The pH of a solution prepared by
Q110: What is the pH of a buffer
Q111: What is the pH of a solution
Q112: Calculate the pH of a buffer that
Q113: A buffer solution contains 0.100 M fluoride
Q114: The pH of a solution prepared by
Q116: Which of the following could be added