Multiple Choice
The concentration of iodide ions in a saturated solution of silver iodide is ________ M.The solubility product constant of AgI is 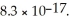
A) 3.8 × 10-11
B) 3.0 × 10-10
C) 9.1 × 10-9
D) 3.5 × 10-9
E) 1.4 × 10-8
Correct Answer:

Verified
Correct Answer:
Verified
Q50: Which one of the following is amphoteric?<br>A)H<sub>2</sub>SO<sub>4</sub><br>B)H<sub>2</sub>O<sub>2</sub><br>C)CO<sub>2</sub><br>D)H<sub>2</sub>O<br>E)NaOH
Q51: In which of the following aqueous solutions
Q52: The addition of HCl and _ to
Q53: Which compound listed below has the smallest
Q54: Which solution has the greatest buffering capacity?<br>A)0.335M
Q56: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The of
Q57: What is the molar solubility of calcium
Q58: 0.78 M Na <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="0.78 M
Q59: The addition of HCl and _ to
Q60: In which aqueous system is <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg"