Multiple Choice
0.78 M Na 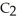
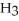
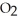 and
and 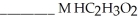 are required to prepare a buffer solution with a pH of 4.40 .The
are required to prepare a buffer solution with a pH of 4.40 .The 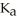 of H
of H 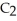
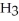
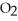 is
is 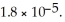
A) 3.5
B) 4.1 × 104
C) 1.7
D) 0.86
E) 0.35
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q53: Which compound listed below has the smallest
Q54: Which solution has the greatest buffering capacity?<br>A)0.335M
Q55: The concentration of iodide ions in a
Q56: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The of
Q57: What is the molar solubility of calcium
Q59: The addition of HCl and _ to
Q60: In which aqueous system is <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg"
Q61: For any buffer system,the buffer capacity depends
Q62: The pH of a solution prepared by
Q63: The addition of KOH and _ to