Multiple Choice
Calculate the pH of a solution that is 0.322 M in nitrous acid ( 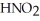 ) and 0.178 M in potassium nitrite (KNO2) .The acid dissociation constant of nitrous acid is 4.50 ×
) and 0.178 M in potassium nitrite (KNO2) .The acid dissociation constant of nitrous acid is 4.50 × 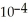 .
.
A) 3.093
B) 3.607
C) 14.26
D) 10.91
E) 4.589
Correct Answer:

Verified
Correct Answer:
Verified
Q44: A solution is prepared by dissolving 0.23
Q45: A 25.0-mL sample of 0.150 M hypochlorous
Q46: A 50.0 mL sample of a solution
Q47: The solubility of slightly soluble salts containing
Q48: A result of the common-ion effect is
Q50: Which one of the following is amphoteric?<br>A)H<sub>2</sub>SO<sub>4</sub><br>B)H<sub>2</sub>O<sub>2</sub><br>C)CO<sub>2</sub><br>D)H<sub>2</sub>O<br>E)NaOH
Q51: In which of the following aqueous solutions
Q52: The addition of HCl and _ to
Q53: Which compound listed below has the smallest
Q54: Which solution has the greatest buffering capacity?<br>A)0.335M