Multiple Choice
A solution is prepared by dissolving 0.23 mol of benzoic acid and 0.27 mol of sodium benzoate in water sufficient to yield 1.00 L of solution.The addition of 0.05 mol of NaOH to this buffer solution causes the pH to increase slightly.The pH does not increase drastically because the NaOH reacts with the ________ present in the buffer solution.The 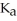 of benzoic acid is 6.3 × 10-5.
of benzoic acid is 6.3 × 10-5.
A) 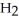 O
O
B) 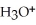
C) benzoate
D) benzoic acid
E) This is a buffer solution: the pH does not change upon addition of acid or base.
Correct Answer:

Verified
Correct Answer:
Verified
Q39: Human blood is considered to be _.<br>A)neutral<br>B)very
Q40: Calculate the maximum concentration (in M)of silver
Q41: Which of the following could be added
Q42: A solution containing which one of the
Q43: The pH of a solution prepared by
Q45: A 25.0-mL sample of 0.150 M hypochlorous
Q46: A 50.0 mL sample of a solution
Q47: The solubility of slightly soluble salts containing
Q48: A result of the common-ion effect is
Q49: Calculate the pH of a solution that