Multiple Choice
A buffer solution with a pH of 4.31 is prepared with 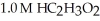 and
and 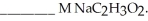 The Ka of H
The Ka of H 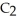
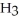
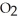 is
is 
A) 0.37
B) 0.74
C) 4.2 × 10-6
D) 8.8 × 10-10
E) 0.18
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: Which of the following could be added
Q6: _ analysis determines how much of a
Q7: The extent of ionization of a weak
Q8: Calculate the maximum concentration (in M)of calcium
Q9: The _ and _ are the principal
Q11: A 25.0 mL sample of a solution
Q12: Which compound listed below has the greatest
Q13: A 25.0 mL sample of 0.723 M
Q14: A solution containing which one of the
Q15: What is the molar solubility of silver