Multiple Choice
How many milliliters of 0.0839 M NaOH are required to titrate 25.0 mL of 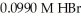 to the equivalence point?
to the equivalence point?
A) 29.5
B) 0.332
C) 4.57
D) 0.208
E) 21.2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q64: Calculate the pH of a solution prepared
Q65: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt=" acid and
Q66: Which one of the following pairs cannot
Q67: The molar solubility of _ is not
Q68: Which is the correct <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="Which
Q70: In which of the following aqueous solutions
Q71: Metal oxides and hydroxides that are relatively
Q72: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The of
Q73: What is the primary buffer system that
Q74: A complex ion is when a metal