Multiple Choice
Calculate the pH of a solution prepared by dissolving 0.250 mol of benzoic acid ( 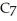
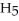
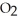 H) and
H) and 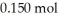 of sodium benzoate (Na
of sodium benzoate (Na 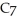
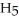
 ) in water sufficient to yield 1.00 L of solution.The
) in water sufficient to yield 1.00 L of solution.The 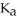 of benzoic acid is
of benzoic acid is 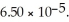
A) 4.409
B) 3.965
C) 10.035
D) 9.591
E) 5.190
Correct Answer:

Verified
Correct Answer:
Verified
Q59: The addition of HCl and _ to
Q60: In which aqueous system is <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg"
Q61: For any buffer system,the buffer capacity depends
Q62: The pH of a solution prepared by
Q63: The addition of KOH and _ to
Q65: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt=" acid and
Q66: Which one of the following pairs cannot
Q67: The molar solubility of _ is not
Q68: Which is the correct <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="Which
Q69: How many milliliters of 0.0839 M NaOH