Multiple Choice
The dissociation energy of N2 is 941 kJ/mol.The maximum wavelength of light that has enough energy per photon to dissociate the N2 molecule is ________ nm.
A) 3137
B) 1.27 × 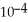
C) 1.27 × 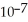
D) 1.27 × 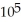
E) 127
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: How can the presence of biodegradable waste
Q21: The most abundant component of air is
Q22: Which of the following is not a
Q23: Which one of the following is produced
Q24: The C-Cl and C-F bond dissociation energies
Q26: Of the noble gases,_ is present in
Q27: The acidic water of a lake (V
Q28: The concentration of ozone in a sample
Q29: Of the compounds below,the one that requires
Q30: The ionization energy of NO is 890