Multiple Choice
The ionization energy of NO is 890 kJ/mol: NO + hv → NO+ + 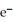 The maximum wavelength of light capable of causing the ionization of NO is ________ nm.
The maximum wavelength of light capable of causing the ionization of NO is ________ nm.
A) 2967
B) 1.35 × 10-4
C) 1.35 × 10-7
D) 134.5
E) 1.35 × 105
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q25: The dissociation energy of N<sub>2</sub> is 941
Q26: Of the noble gases,_ is present in
Q27: The acidic water of a lake (V
Q28: The concentration of ozone in a sample
Q29: Of the compounds below,the one that requires
Q31: Incomplete combustion of carbon-containing materials occurs when
Q32: In the presence of oxygen,the nitrogen present
Q33: The concept that radiowave propagation was affected
Q34: In the purification of drinking water,the filtered
Q35: In the past,CFCs were used in which