Multiple Choice
Given the thermodynamic data in the table below,calculate the equilibrium constant (at 298 K) for the reaction: 2 SO2 (g) + O2 (g)  2 SO3 (g)
2 SO3 (g) 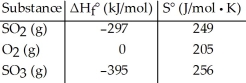
A) 2.40 × 1024
B) 1.06
C) 1.95
D) 3.82 × 1023
E) More data are needed.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q44: The standard Gibbs free energy of formation
Q45: For a given reaction,ΔS = +69.0 J/mol∙K,and
Q46: The more negative ΔG° is for a
Q47: The combustion of ethene in the presence
Q48: Which of the following has the largest
Q50: ΔS is positive for the reaction _.<br>A)2
Q51: The value of ΔS° for the reaction
Q52: ΔS is negative for the reaction _.<br>A)Sr(NO<sub>3</sub>)<sub>2</sub>
Q53: Which reaction produces an increase in the
Q54: At what temperature in Kelvin will a