Multiple Choice
At what temperature in Kelvin will a reaction have 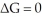 ? ΔH = -24.2 kJ/mol and ΔS = -55.5 J/K-mol and assume both do not vary with temperature.
? ΔH = -24.2 kJ/mol and ΔS = -55.5 J/K-mol and assume both do not vary with temperature.
A) 2.29
B) 2293
C) 298
D) 436
E) 0.436
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q49: Given the thermodynamic data in the table
Q50: ΔS is positive for the reaction _.<br>A)2
Q51: The value of ΔS° for the reaction
Q52: ΔS is negative for the reaction _.<br>A)Sr(NO<sub>3</sub>)<sub>2</sub>
Q53: Which reaction produces an increase in the
Q55: Which one of the following statements is
Q56: The value of ΔG° at 25 °C
Q57: The melting of a substance at its
Q58: The value of ΔG° at 25 °C
Q59: For a given reaction with ΔS =