Multiple Choice
The value of ΔG° at 261.0 °C for the formation of phosphorous trichloride from its constituent elements, 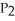 (g) +
(g) + 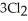 (g) →
(g) → 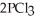 (g) is ________ kJ/mol.At 25.0 °C for this reaction,ΔH° is -720.5 kJ/mol,ΔG° is
(g) is ________ kJ/mol.At 25.0 °C for this reaction,ΔH° is -720.5 kJ/mol,ΔG° is 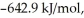 and
and 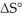 is
is 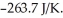
A) -579.6
B) 6.81 × 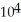
C) 1.40 × 105
D) -651.7
E) -861.4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q75: Consider the reaction: FeO (s)+ Fe (s)+
Q76: The value of ΔH° for the decomposition
Q77: Which of the following statements is true?<br>A)Processes
Q78: Which of the following has the largest
Q79: With thermodynamics,one cannot determine _.<br>A)the speed of
Q81: A reaction that is not spontaneous at
Q82: The value of ΔS° for the oxidation
Q83: The value of ΔG° at 25 °C
Q84: The combustion of hydrogen in the presence
Q85: A reaction that is spontaneous as written