Multiple Choice
Consider the reaction: FeO (s) + Fe (s) + O2 (g) → Fe2O3 (s)
Given the following table of thermodynamic data, 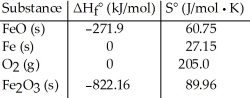 determine the temperature (in °C) at which the reaction is nonspontaneous.
determine the temperature (in °C) at which the reaction is nonspontaneous.
A) below 618.1
B) above 2438
C) above 756.3
D) below 2438
E) This reaction is spontaneous at all temperatures.
Correct Answer:

Verified
Correct Answer:
Verified
Q70: The value of ΔG° at 25 °C
Q71: The value of ΔG° for a reaction
Q72: Consider the reaction: Ag<sup>+</sup> (aq)+ Cl<sup>-</sup> (aq)→
Q73: The equilibrium constant for the following reaction
Q74: Which one of the following processes produces
Q76: The value of ΔH° for the decomposition
Q77: Which of the following statements is true?<br>A)Processes
Q78: Which of the following has the largest
Q79: With thermodynamics,one cannot determine _.<br>A)the speed of
Q80: The value of ΔG° at 261.0 °C