Multiple Choice
The equilibrium constant for the following reaction is 3.0 × 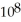 at 25 °C.
at 25 °C. 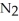 (g) + 3
(g) + 3 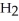 (g)
(g)  2N
2N 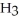 (g) The value of ΔG° for this reaction is ________ kJ/mol.
(g) The value of ΔG° for this reaction is ________ kJ/mol.
A) 22
B) -4.1
C) 4.1
D) -48
E) -22
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q68: Which of the following has the largest
Q69: At what temperature (in K)will a reaction
Q70: The value of ΔG° at 25 °C
Q71: The value of ΔG° for a reaction
Q72: Consider the reaction: Ag<sup>+</sup> (aq)+ Cl<sup>-</sup> (aq)→
Q74: Which one of the following processes produces
Q75: Consider the reaction: FeO (s)+ Fe (s)+
Q76: The value of ΔH° for the decomposition
Q77: Which of the following statements is true?<br>A)Processes
Q78: Which of the following has the largest