Multiple Choice
The standard cell potential (E°cell) of the reaction below is +1.34 V.The value of 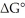 for the reaction is ________ kJ/mol. 3 Cu (s) + 2 MnO4- (aq) + 8H+ (aq) → 3 Cu2+ (aq) + 2 MnO2 (s) + 4 H2O (l)
for the reaction is ________ kJ/mol. 3 Cu (s) + 2 MnO4- (aq) + 8H+ (aq) → 3 Cu2+ (aq) + 2 MnO2 (s) + 4 H2O (l)
A) -24.3
B) +259
C) -259
D) +776
E) -776
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: What is being reduced at the cathode
Q34: Which of the following reactions will occur
Q35: The standard cell potential (E°<sub>cell</sub>)for the voltaic
Q36: The standard emf for the cell using
Q37: The standard cell potential (E°)of a voltaic
Q39: A voltaic cell is constructed with two
Q40: At constant _ and _ the Gibbs
Q41: 1V = _.<br>A)1 amp ∙ s<br>B)1 J/s<br>C)96485
Q42: How many minutes will it take to
Q43: How many grams of copper will be