Multiple Choice
A voltaic cell is constructed with two silver-silver chloride electrodes,where the half-reaction is AgCl (s) + 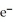 → Ag (s) +
→ Ag (s) + 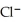 (aq) E° = +0.222 V
(aq) E° = +0.222 V
The concentrations of chloride ion in the two compartments are 0.0100 M and 1.55 M,respectively.At 25 °C,the cell emf is ________ V.
A) 0.216
B) 0.130
C) 0.00143
D) 34.4
E) 0.228
Correct Answer:

Verified
Correct Answer:
Verified
Q34: Which of the following reactions will occur
Q35: The standard cell potential (E°<sub>cell</sub>)for the voltaic
Q36: The standard emf for the cell using
Q37: The standard cell potential (E°)of a voltaic
Q38: The standard cell potential (E°<sub>cell</sub>)of the reaction
Q40: At constant _ and _ the Gibbs
Q41: 1V = _.<br>A)1 amp ∙ s<br>B)1 J/s<br>C)96485
Q42: How many minutes will it take to
Q43: How many grams of copper will be
Q44: Which of the following reactions will occur