Multiple Choice
Which element is reduced in the reaction below?
Fe2+ + H+ + Cr2O72- → Fe3+ + Cr3+ + 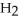 O
O
A) Cr
B) Fe
C) H
D) O
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: The standard cell potential (E°<sub>cell</sub>)for the voltaic
Q16: The standard cell potential ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg"
Q17: Corrosion of iron is retarded by _.<br>A)the
Q18: In the electrochemical cell using the redox
Q19: _ is the reducing agent in the
Q21: _ is the reducing agent in the
Q22: In a voltaic cell,electrons flow from the
Q23: In a lead-acid battery,the electrodes are consumed.In
Q24: What is the oxidation number of chromium
Q25: One of the differences between a voltaic