Multiple Choice
The standard cell potential ( 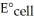 ) for the reaction below is +1.10 V.The cell potential for this reaction is ________ V when the concentration of
) for the reaction below is +1.10 V.The cell potential for this reaction is ________ V when the concentration of 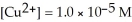 and
and 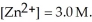 Zn (s) +
Zn (s) + 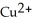 (aq) → Cu (s) +
(aq) → Cu (s) + 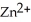 (aq)
(aq)
A) 1.42
B) 1.26
C) 0.94
D) 0.78
E) 1.10
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: In a voltaic cell electrons flow from
Q12: A voltaic cell is constructed with two
Q13: What is the oxidation number of bromine
Q14: Which of the halogens in Table 20.1
Q15: The standard cell potential (E°<sub>cell</sub>)for the voltaic
Q17: Corrosion of iron is retarded by _.<br>A)the
Q18: In the electrochemical cell using the redox
Q19: _ is the reducing agent in the
Q20: Which element is reduced in the reaction
Q21: _ is the reducing agent in the