Multiple Choice
A voltaic cell is constructed with two 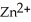 -Zn electrodes,where the half-reaction is
-Zn electrodes,where the half-reaction is 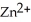 +
+ 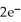 → Zn (s) E° = -0.763 V The concentrations of zinc ion in the two compartments are 4.50 M and 1.11 ×
→ Zn (s) E° = -0.763 V The concentrations of zinc ion in the two compartments are 4.50 M and 1.11 × 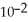 M,respectively.The cell emf is ________ V.
M,respectively.The cell emf is ________ V.
A) -1.88 × 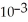
B) -309
C) 0.0772
D) 0.154
E) -0.761
Correct Answer:

Verified
Correct Answer:
Verified
Q7: Which transformation below is an example of
Q8: The standard cell potential ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg"
Q9: Which substance is the oxidizing agent in
Q10: Which substance is the oxidizing agent in
Q11: In a voltaic cell electrons flow from
Q13: What is the oxidation number of bromine
Q14: Which of the halogens in Table 20.1
Q15: The standard cell potential (E°<sub>cell</sub>)for the voltaic
Q16: The standard cell potential ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg"
Q17: Corrosion of iron is retarded by _.<br>A)the