Multiple Choice
The average mass of an atom of sulfur is 5.33 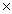 10 -23 g.How many sulfur atoms are present in a 100.0g sample of sulfur?
10 -23 g.How many sulfur atoms are present in a 100.0g sample of sulfur?
A) 5.33 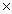 10 -21
10 -21
B) 1.88 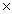 10 -21
10 -21
C) 5.33 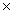 10 24
10 24
D) 1.88 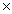 10 24
10 24
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: An isotope of sodium has the atomic
Q10: Which subatomic particle contributes least to the
Q11: A substance has 15 protons,16 neutrons,and 15
Q12: In isotopic notation atomic number is represented
Q13: The name of the isotope containing one
Q15: An atom of zinc has a mass
Q16: The majority of the volume of an
Q17: Particles with which electric charges will attract
Q18: An atom of gallium has a mass
Q19: Rutherford's gold foil experiment proved that the