Multiple Choice
An atom of gallium has a mass of 69.72 amu.On of its isotopes, 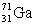 ,has a 39.89% abundance.Since 1 amu = 1.6606 x 10-24 g,how many atoms of this gallium isotope would be present in a 1.00 mg sample of gallium?
,has a 39.89% abundance.Since 1 amu = 1.6606 x 10-24 g,how many atoms of this gallium isotope would be present in a 1.00 mg sample of gallium?
A) 3.445 x 1018 atoms
B) 8.637 x 1018 atoms
C) 2.165 x 1019 atoms
D) 9.501 x 1018 atoms
Correct Answer:

Verified
Correct Answer:
Verified
Q13: The name of the isotope containing one
Q14: The average mass of an atom of
Q15: An atom of zinc has a mass
Q16: The majority of the volume of an
Q17: Particles with which electric charges will attract
Q19: Rutherford's gold foil experiment proved that the
Q20: Atom A has 5 protons and 6
Q21: Which contains the largest number of neutrons?<br>A)C-12<br>B)P-31<br>C)Br-80<br>D)Sr-90
Q22: Which isotope contains the same number of
Q23: How many protons are in a neutral