Multiple Choice
A solution of a strong base has a pH of 11.The concentration of hydronium ions in this solution is
A) 11 M
B) 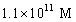
C) 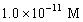
D) 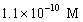
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: An amphoteric substance is one that<br>A)may react
Q16: The hydroxide ion is responsible for the
Q17: Which is the pH of a solution
Q18: A 400.g sample of aluminum sulfate is
Q19: An aqueous solution is 12.5% in Na<sub>2</sub>CO<sub>3</sub>
Q21: Which is a strong acid?<br>A)HNO<sub>3</sub><br>B)HNO<sub>2</sub><br>C)HClO<br>D)HClO<sub>2</sub>
Q22: A 0.1 M HNO<sub>3</sub> solution and a
Q23: In the following reaction, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4036/.jpg" alt="In
Q24: A solution is prepared by adding 35.5
Q25: What is the concentration of an HCl