Multiple Choice
An aqueous solution is 12.5% in Na2CO3 and has a density of 1.19 g/mL.What is the molarity of this solution?
A) 0.117 M
B) 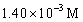
C) 14.9 M
D) 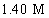
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: What are the spectator ions when hydrochloric
Q15: An amphoteric substance is one that<br>A)may react
Q16: The hydroxide ion is responsible for the
Q17: Which is the pH of a solution
Q18: A 400.g sample of aluminum sulfate is
Q20: A solution of a strong base has
Q21: Which is a strong acid?<br>A)HNO<sub>3</sub><br>B)HNO<sub>2</sub><br>C)HClO<br>D)HClO<sub>2</sub>
Q22: A 0.1 M HNO<sub>3</sub> solution and a
Q23: In the following reaction, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4036/.jpg" alt="In
Q24: A solution is prepared by adding 35.5