Multiple Choice
Which of the following correctly arranges 1.00 M solutions of the strong electrolytes in order of increasing boiling point (lowest to highest) ?
A) 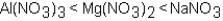
B) 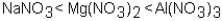
C) 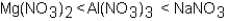
D) All have the same boiling point.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: There is a 12 M aqueous HCl
Q9: Ionic compounds are generally insoluble in non-polar
Q16: Which compound is most soluble in a
Q21: Which of the following pairs can produce
Q40: The spores from many molds produce an
Q57: How many moles of Na<sub>2</sub>CO<sub>3</sub> are
Q60: A solution is prepared at 75 °C
Q66: One test to determine if a mixture
Q79: Weight-volume percentage solutions must be made in
Q97: You have a patient who is suffering