Multiple Choice
A solution is prepared at 75 °C by dissolving 50.0 g of A in 100 g of water.Which of the following correctly classifies this solution based on the solubility chart for A given below? 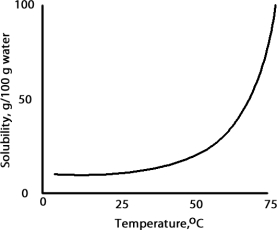
A) saturated
B) unsaturated
C) supersaturated
D) immiscible
Correct Answer:

Verified
Correct Answer:
Verified
Q9: Ionic compounds are generally insoluble in non-polar
Q21: Which of the following pairs can produce
Q22: The primary intermolecular attractions between CH<sub>3</sub>-OH and
Q40: The spores from many molds produce an
Q49: When an ionic substance dissolves, the solvated
Q56: Which of the following correctly arranges 1.00
Q57: How many moles of Na<sub>2</sub>CO<sub>3</sub> are
Q66: One test to determine if a mixture
Q85: Changes in boiling point, freezing point, and
Q95: The rate of osmosis<br>A)can be increased by