Multiple Choice
One of the main problems with the Bohr model of the hydrogen atom when compared with the results of the methods of quantum mechanics used to describe atoms,was that the Bohr model predicted
A) the ground state angular momentum was L = 1 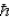 .
.
B) the frequency of the radiation emitted when an electron "jumps" from one allowed orbit to another was hf = Ei − Ef.
C) the potential energy function for the hydrogen atom was given by V(r) = −ke2/r.
D) the energy of the ground state of the hydrogen atom was En = −13.6 eV.
Correct Answer:

Verified
Correct Answer:
Verified
Q19: Forbidden transitions and selection rules suggest that<br>A)
Q34: A hydrogen atom emits a photon of
Q44: What angle does the orbital angular momentum
Q46: A hydrogen atom in the 4f state
Q48: If P(r)is the radial probability density function
Q51: Quantum physics agrees with the classical physics
Q52: In an atom that has an electron
Q53: The energy needed to remove an electron
Q56: In the operation of a laser<br>A) stimulated
Q59: A headwaiter at a restaurant decides to