Multiple Choice
If P(r) is the radial probability density function for an electron in the ground state of a hydrogen atom,the most probable value for r can be found from
A) dP/dt
B) dP/dr
C) 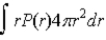
D) 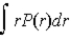
E) d2P/dr2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: Forbidden transitions and selection rules suggest that<br>A)
Q42: Aline says that the magnetic moment of
Q44: What angle does the orbital angular momentum
Q46: A hydrogen atom in the 4f state
Q49: One of the main problems with the
Q51: Quantum physics agrees with the classical physics
Q52: In an atom that has an electron
Q53: The energy needed to remove an electron
Q56: In the operation of a laser<br>A) stimulated
Q59: A headwaiter at a restaurant decides to