Multiple Choice
When using the Pauli Exclusion Principle,we assume the particle's spin angular momentum is of magnitude
A) 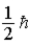
B) 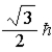
C) 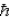
D) ± 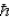
E) 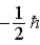
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: Forbidden transitions and selection rules suggest that<br>A)
Q31: The number of states in the He<sup>+</sup>
Q34: A hydrogen atom emits a photon of
Q49: One of the main problems with the
Q51: Quantum physics agrees with the classical physics
Q52: The allowed values of n for the
Q52: In an atom that has an electron
Q53: The energy needed to remove an electron
Q54: Rubidium (Z = 37) and potassium (Z
Q55: Which of the following statements is true?<br>A)