Multiple Choice
If the average kinetic energy of the molecules of a monatomic ideal gas at 55°C is doubled,what is the resulting temperature of the gas?
A) It becomes 55× 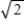 °C.
°C.
B) It becomes 656°C.
C) It becomes 110°C.
D) It becomes 383°C.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: What happens to a volume of water
Q3: Two ideal gases,X and Y,are thoroughly mixed
Q4: The length of a 25-m wire increases
Q5: A rectangular steel plate with dimensions of
Q6: At 22.0°C an aluminum ring has an
Q7: What is the mass of a mole
Q8: An automobile gas tank is filled to
Q9: The temperature of a quantity of ideal
Q10: The sulfur hexafluoride molecule consists of one
Q11: 13.0 g of water in a 3.25-L