Multiple Choice
Suppose that a certain atom possesses only four distinct energy levels. Assuming that all transitions between levels are possible, how many spectral lines will this atom exhibit? 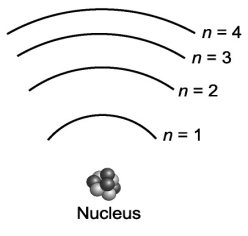
A) three
B) four
C) six
D) unlimited
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: Thousands of magnetic marbles are thrown into
Q28: In some instances electromagnetic radiation behaves like
Q44: A one ounce Ping-Pong ball is pushed
Q66: Does a shell have to contain electrons
Q85: How is it possible to deduce the
Q106: Would you use a physical model or
Q114: Which of the following statements is true
Q125: Which of the above has the highest
Q126: Consider the various frequencies of the three
Q128: What does the following element description actually