Multiple Choice
Consider the various frequencies of the three photons emitted from the following three individual electron transitions in the figure below: n=3 to n=2; n=2 to n=1; n=3 to n=1. These transitions would produce three spectral lines in a spectroscope. If the energy spacing between the levels were equal, would this affect the number of spectral lines? 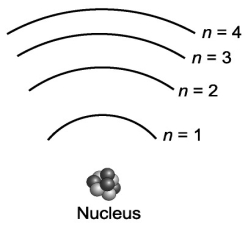
A) Yes, two otherwise separate lines would converge into a single more intense line.
B) No, but the spacing between the spectral lines would change.
C) Yes, two otherwise separate lines would converge into a single less intense line.
D) No, but some would then require a prism in order to be seen.
Correct Answer:

Verified
Correct Answer:
Verified
Q3: Thousands of magnetic marbles are thrown into
Q15: The nucleus of an electrically neutral iron
Q16: From which of the following atoms would
Q28: In some instances electromagnetic radiation behaves like
Q85: How is it possible to deduce the
Q106: Would you use a physical model or
Q111: An atom absorbs or emits only particular
Q114: Which of the following statements is true
Q128: What does the following element description actually
Q129: Suppose that a certain atom possesses only