Multiple Choice
Why is calcium chloride, 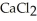 , more effective at melting ice than sodium chloride, NaCl?
, more effective at melting ice than sodium chloride, NaCl?
A) Calcium ions are both larger and more highly charged and thus more effective in breaking the ice crystal structure.
B) Calcium ions are smaller than sodium ions and thus more able to get in the way of the water molecules attempting to form the solid state lattice.
C) Calcium chloride produces three ion particles while sodium chloride produces only two. Greater numbers of ions are more effective at decreasing the number of water molecules entering the solid phase.
D) Sodium ion is a natural water softener and therefore not as effective in inhibiting the formation of the solid state of water from the liquid.
Correct Answer:

Verified
Correct Answer:
Verified
Q3: Like water, hydrogen fluoride, HF, and ammonia,
Q9: What would happen to the oceans if
Q11: If the specific heat of aluminum is
Q15: Heat is given off by a substance
Q18: What is the boiling temperature of a
Q21: Why do ice cubes get smaller when
Q35: If it takes 200 J to raise
Q41: From the diagram above describing the phase
Q63: Which of the above liquids would most
Q76: Water coming out of volcanic sea vents