Multiple Choice
Like water, hydrogen fluoride, HF, and ammonia, N 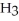 , have relatively high boiling points. Explain.
, have relatively high boiling points. Explain.
A) Even though HF and N 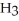 have ionic bonds, like water they have a strong attraction for themselves.
have ionic bonds, like water they have a strong attraction for themselves.
B) Since these are all relatively small molecules, they can compact more tightly together, and will require more energy to be separated from each other.
C) The polar molecules of each of these materials have relatively strong attractions for themselves, which translates to relatively high boiling points.
D) Since these molecules interact with each other in long chains, they have many regions of attraction and are held together relatively tightly, thus, they are harder to pull apart when boiling.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Consider a lake that is uniformly 10°C.
Q7: Why is calcium chloride, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6000/.jpg" alt="Why
Q9: What would happen to the oceans if
Q11: If the specific heat of aluminum is
Q15: Heat is given off by a substance
Q18: What is the boiling temperature of a
Q21: Why do ice cubes get smaller when
Q35: If it takes 200 J to raise
Q41: From the diagram above describing the phase
Q76: Water coming out of volcanic sea vents