Multiple Choice
Identify the acid or base behavior of each substance in these reactions: 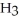
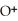 +
+ 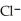 ⇌
⇌ 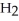 O + HCl
O + HCl
A) 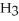
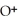 acts as an acid,
acts as an acid, 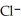 acts as a base,
acts as a base, 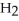 O acts an acid, HCl acts as a base.
O acts an acid, HCl acts as a base.
B) 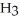
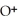 acts as an base,
acts as an base, 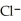 acts as a acid,
acts as a acid, 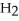 O acts an acid, HCl acts as a base.
O acts an acid, HCl acts as a base.
C) 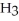
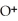 acts as an acid,
acts as an acid, 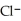 acts as a base,
acts as a base, 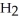 O acts an base, HCl acts as a acid.
O acts an base, HCl acts as a acid.
D) 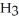
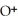 acts as an base,
acts as an base, 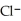 acts as a acid,
acts as a acid, 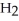 O acts an base, HCl acts as an acid.
O acts an base, HCl acts as an acid.
Correct Answer:

Verified
Correct Answer:
Verified
Q6: The amphoteric reaction between two water molecules
Q9: What does the value of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6000/.jpg"
Q11: Along with the pH scale, there is
Q69: Can an acid and a base react
Q91: A buffer solution can neutralize _.<br>A)strong acids
Q99: Carbon dioxide reacts with water to form
Q114: How does a base like NH<sub>3</sub> raise
Q127: Lakes lying in granite basins,such as those
Q140: For the following reaction,identify whether the compound
Q144: Water is formed from the reaction of