Multiple Choice
The amphoteric reaction between two water molecules is endothermic, which means that this reaction requires the input of energy in order to proceed: Energy + 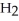 O +
O + 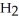 O →
O → 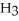
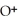 + O
+ O 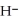 The warmer the temperature of the water, the more thermal energy is available for this reaction, and the more hydronium and hydroxide ions are formed. Based on this information, should the value of
The warmer the temperature of the water, the more thermal energy is available for this reaction, and the more hydronium and hydroxide ions are formed. Based on this information, should the value of 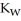 be expected to increase, decrease, or stay the same with increasing temperature?
be expected to increase, decrease, or stay the same with increasing temperature?
A) The value of 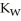 will stay the same, since it is a constant.
will stay the same, since it is a constant.
B) The value of 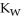 will decrease, since there are more hydronium ions than hydroxide ions.
will decrease, since there are more hydronium ions than hydroxide ions.
C) The value of 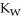 will increase since the rise in temperature allows for a higher concentration of both ions.
will increase since the rise in temperature allows for a higher concentration of both ions.
D) The value of 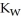 will decrease since there are more hydroxide ions than hydronium ions.
will decrease since there are more hydroxide ions than hydronium ions.
Correct Answer:

Verified
Correct Answer:
Verified
Q3: Identify the acid or base behavior of
Q9: What does the value of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6000/.jpg"
Q11: Along with the pH scale, there is
Q69: Can an acid and a base react
Q91: A buffer solution can neutralize _.<br>A)strong acids
Q99: Carbon dioxide reacts with water to form
Q114: How does a base like NH<sub>3</sub> raise
Q127: Lakes lying in granite basins,such as those
Q140: For the following reaction,identify whether the compound
Q144: Water is formed from the reaction of