Multiple Choice
Identify the acid or base behavior of each substance in these reactions: HS 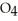
 +
+ 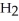 O ⇌ O
O ⇌ O 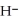 +
+ 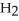 S
S 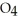
A) HS 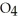
 acts as an acid,
acts as an acid, 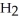 O acts as a base, O
O acts as a base, O 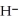 acts as an acid,
acts as an acid, 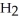 S
S 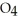 acts as a base.
acts as a base.
B) HS 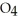
 acts as an base,
acts as an base, 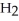 O acts as a acid, O
O acts as a acid, O 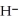 acts as an acid,
acts as an acid, 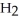 S
S 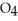 acts as a base.
acts as a base.
C) HS 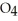
 acts as an acid,
acts as an acid, 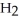 O acts as a base, O
O acts as a base, O 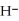 acts as an base,
acts as an base, 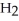 S
S 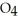 acts as a acid.
acts as a acid.
D) HS 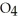
 acts as an base,
acts as an base, 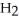 O acts as a acid, O
O acts as a acid, O 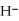 acts as an base,
acts as an base, 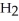 S
S 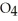 acts as a acid.
acts as a acid.
Correct Answer:

Verified
Correct Answer:
Verified
Q20: What is the relationship between the hydroxide
Q23: For the following acid-base reaction,identify what is
Q29: Acetic acid, shown below, has 4 hydrogen
Q30: What is pH?<br>A)It is a numerical scale
Q74: Which of the following compounds could never
Q80: If you had a 1 M solution
Q96: The hydroxide ion, HO<sup>-</sup>, is a _.<br>A)unique
Q105: If you were to increase the pH
Q106: Adding more carbon dioxide to the atmosphere
Q154: Pour vinegar onto beach sand from the