Multiple Choice
Acetic acid, shown below, has 4 hydrogen atoms-one bonded to an oxygen and three bonded to a carbon. When this molecule behaves as an acid, it donates only the hydrogen bonded to the oxygen. The hydrogens bonded to the carbon remain intact. Why? 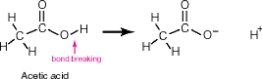
A) The oxygen is much better at accommodating a negative charge than is carbon.
B) The hydrogen attached to the oxygen extends farther away from the center of the molecule.
C) The carbon is bonded to three hydrogens while the oxygen is bonded to only one hydrogen.
D) The oxygen within acetic acid has two lone pairs of electrons that help to destabilize the oxygen-hydrogen bond.
Correct Answer:

Verified
Correct Answer:
Verified
Q20: What is the relationship between the hydroxide
Q23: For the following acid-base reaction,identify what is
Q26: Identify the acid or base behavior of
Q30: What is pH?<br>A)It is a numerical scale
Q74: Which of the following compounds could never
Q80: If you had a 1 M solution
Q83: If you had a 1 M solution
Q105: If you were to increase the pH
Q122: Which of the following statements describes a
Q140: For the following reaction,identify whether the compound