Multiple Choice
The main component of bleach is sodium hypochlorite, NaOCl, which consists of sodium ions, Na⁺, and hypochlorite ions, 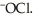 What products are formed when this compound is reacted with the hydrochloric acid, HCl, of toilet bowl cleaner?
What products are formed when this compound is reacted with the hydrochloric acid, HCl, of toilet bowl cleaner?
A) The products are NaCl,  , and
, and 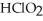 .
.
B) The products are NaOH, 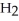 O, and
O, and 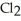
C) The products are NaCl and HOCl.
D) The products are NaOH, 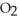 , and
, and 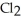
Correct Answer:

Verified
Correct Answer:
Verified
Q26: As the pH increases,the hydroxide ion concentration<br>A)goes
Q27: If the pH of a solution is
Q73: Which of the following reactions illustrates an
Q89: What is the source of acid rain?<br>A)Acid
Q92: If you had a 1 M solution
Q105: Does the hydride ion, H⁻, tend to
Q111: Which should be a stronger base: ammonia,
Q115: How readily an acid donates a hydrogen
Q120: What happens to the pH of a
Q121: An acid and a base react to