Multiple Choice
How readily an acid donates a hydrogen ion is a function of how well the acid is able to accommodate the resulting negative charge it gains after donating. Which should be the stronger acid: water or hypochlorous acid? Why? 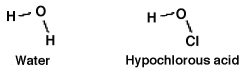
A) Water, because O-H would accommodate the resulting negative charge better than O-Cl.
B) Hypochlorous acid, because the resulting negative charge is spread over a greater number of atoms.
C) Neither, since both molecules are "monoprotic" and donate the same H+ ion, making their the same.
D) Water, because the hydrogen bonding in water gives it the edge in the ability to accommodate the resulting negative charge.
Correct Answer:

Verified
Correct Answer:
Verified
Q26: As the pH increases,the hydroxide ion concentration<br>A)goes
Q79: Which of the following items would you
Q89: What is the source of acid rain?<br>A)Acid
Q100: Qualitatively, what happens to the hydroxide ion
Q110: The main component of bleach is sodium
Q111: Which should be a stronger base: ammonia,
Q119: When a hydronium ion concentration equals 1
Q120: What happens to the pH of a
Q140: For the following reaction,identify whether the compound
Q174: Why might an area with a large