Multiple Choice
What results when water, 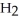 O, reacts with sulfur dioxide, S
O, reacts with sulfur dioxide, S 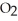 ?
?
A) 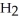 O + S
O + S 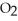 →
→ 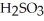 (aq)
(aq)
B) 2 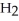 O + 2 S
O + 2 S 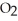 → 2
→ 2 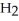
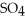 (aq) +
(aq) + 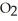 (g)
(g)
C) 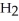 O + S
O + S 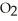 → S
→ S 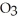 (g) +
(g) + 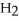 (g)
(g)
D) 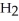 O + S
O + S 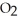 → No Reaction
→ No Reaction
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q45: When the hydronium ion concentration equals 1
Q57: Which of the following solutions is the
Q61: When the pH of a solution is
Q62: How might warmer oceans accelerate global warming?<br>A)
Q63: At what point will a buffer solution
Q65: Some molecules are able to stabilize a
Q72: Cutting back on the pollutants that cause
Q78: Suggest why people once washed their hands
Q138: According to the following reaction,which molecule is
Q156: Which of the following statements describes an