Multiple Choice
At what point will a buffer solution cease to moderate changes in pH?
A) when waters equilibrium constant 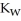 has been reached
has been reached
B) when the weak acid and the weak base salt concentration are equal
C) when the acting buffering component is all neutralized
D) only after all the acid or the base that needs to be buffered has been added
Correct Answer:

Verified
Correct Answer:
Verified
Q55: What would the concentration of H<sub>3</sub>O<sup>+</sup> be
Q57: Which of the following solutions is the
Q61: When the pH of a solution is
Q62: How might warmer oceans accelerate global warming?<br>A)
Q65: Some molecules are able to stabilize a
Q66: What results when water, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6000/.jpg" alt="What
Q77: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6597/.jpg" alt=" -Which of the
Q117: A weak acid is added to a
Q138: According to the following reaction,which molecule is
Q156: Which of the following statements describes an