Multiple Choice
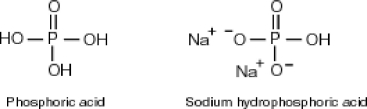
Why is phosphoric acid, 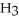
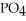 , a stronger acid than disodium hydrogen phosphate,
, a stronger acid than disodium hydrogen phosphate, 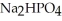 ?
? 
A) Phosphoric acid has three acidic hydrogens which makes it three times as acidic.
B) Some of the released sodium ions in 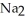 HP
HP 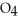 form NaOH (a base) , which decreases the acidity of the
form NaOH (a base) , which decreases the acidity of the 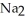 HP
HP 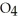 .
.
C) Phosphoric acid dissociates 100% in water whereas 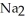 HP
HP 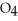 only dissociates about 50%.
only dissociates about 50%.
D) None of the above accurately describes why phosphoric acid is a stronger acid than disodium hydrogen phosphate.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Which of the following solutions is the
Q23: If the oceans became acidic, they would
Q58: Why is carbon dioxide able to be
Q64: When the hydronium ion concentration equals 10
Q82: If the pH of a solution is
Q94: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6597/.jpg" alt=" -Which of the
Q95: What would be the concentration of hydronium
Q98: What atom in the hydronium ion, H<sub>3</sub>O⁺,
Q142: For the following acid-base reaction,identify which salt
Q170: According to the following reaction,which molecule is