Multiple Choice
What would be the concentration of hydronium ions in a solution that had a pH = -3? Why would such a solution be impossible to prepare?
A) Concentration of 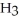
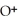 = 3 M. It is impossible because it is hard to measure such a small amount of hydronium ions accurately.
= 3 M. It is impossible because it is hard to measure such a small amount of hydronium ions accurately.
B) Concentration of 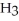
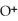 = 30 M. It is impossible because pH can not be negative.
= 30 M. It is impossible because pH can not be negative.
C) Concentration of 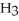
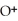 =
= 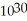 M. It is impossible because any container used to hold the acid would immediately corrode.
M. It is impossible because any container used to hold the acid would immediately corrode.
D) Concentration of 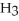
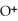 =
= 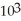 M. It is impossible because only so much acid can dissolve in water before the solution becomes saturated.
M. It is impossible because only so much acid can dissolve in water before the solution becomes saturated.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Which of the following solutions is the
Q22: What is a base?<br>A)anything that accepts a
Q23: If the oceans became acidic, they would
Q25: If we were to lower our rate
Q58: Why is carbon dioxide able to be
Q64: When the hydronium ion concentration equals 10
Q82: If the pH of a solution is
Q93: Why is phosphoric acid, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6000/.jpg" alt="Why
Q98: What atom in the hydronium ion, H<sub>3</sub>O⁺,
Q142: For the following acid-base reaction,identify which salt