Multiple Choice
Why are aqueous solutions of highly charged metal ions, such as 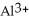 , usually acidic?
, usually acidic?
A) The highly charged 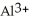 ion strips hydrogen ions away from water as if forms
ion strips hydrogen ions away from water as if forms 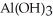 and releases them into the solution causing the solution to become acidic.
and releases them into the solution causing the solution to become acidic.
B) As the water molecule binds to the aluminum ion, the H-O bond becomes more polar. This means that the water molecule is more readily able to form hydrogen ions, which makes the solution acidic.
C) False! 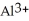 ions tend to make aqueous solutions basic since they pull the OH⁻ ion from the water molecule.
ions tend to make aqueous solutions basic since they pull the OH⁻ ion from the water molecule.
D) The formation of the oxide 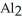
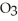 releases hydrogen ions to the aqueous solution making it acidic.
releases hydrogen ions to the aqueous solution making it acidic.
Correct Answer:

Verified
Correct Answer:
Verified
Q7: According to the following reaction,which molecule is
Q21: What happens to the pH of soda
Q37: Which of the following statements about buffers
Q64: When the hydronium ion concentration equals 10
Q82: Sodium hydroxide is added to a buffer
Q94: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6597/.jpg" alt=" -Which of the
Q137: Which of the following statements describes a
Q157: What would be the best explanation for
Q170: According to the following reaction,which molecule is
Q175: For the following acid-base reaction,identify what is